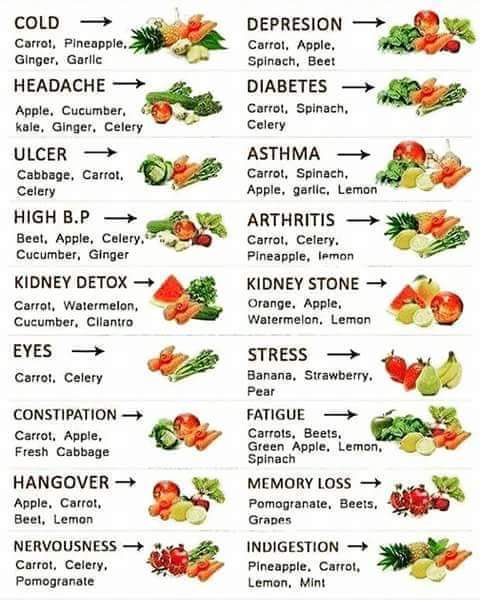
Healthy Choices
Baked Salmon With Caribbean Fruit Salsa
Prep: 5 min. Cook: 25 min. Other: 2 hrs. A whole salmon fillet makes a dramatic presentation. Ask the butcher at your grocery store to remove salmon skin.
Ingredients...
1 (3-pound) whole skinless salmon fillet 1 tablespoon Caribbean jerk seasoning* 1½ tablespoons olive oil Caribbean Fruit Salsa Garnish: lime wedges
Directions...
Place salmon fillet in a roasting pan; sprinkle evenly on 1 side with jerk seasoning. Drizzle with oil. Cover and chill 2 hours.
Bake salmon at 350° for 20 to 25 minutes or until fish flakes with a fork.
Serve with Caribbean Fruit Salsa. Garnish, if desired.
Yield: 8 servings. Per serving: Calories 346 (43% from fat); Fat 16.4g (sat 2.5g, mono 7g, poly 5.4g); Protein 39.1g; Carb 9.2g; Fiber 1.4g; Chol 108mg; Iron 1.8mg; Sodium 192mg; Calc 35mg *
Substitute Jamaican jerk seasoning, if desired. Caribbean jerk seasoning has a hint of sweetness.
Caribbean Fruit Salsa
Prep: 20 min. Other: 2 hrs.
This salsa’s also great as an appetizer served with tortilla chips.
Ingredients...
1 mango (about ½ pound), peeled and diced* 1 papaya (about ½ pound), peeled and diced* 1 medium-size red bell pepper, diced 1 medium-size green bell pepper, diced 1 cup diced fresh pineapple 1 small red onion, diced 3 tablespoons chopped fresh parsley 2 tablespoons fresh lime juice 1 tablespoon olive oil
Directions...
Stir together all ingredients. Cover and chill at least 2 hours.
Yield: 5 cups. Per ¼ cup: Calories 24 (30% from fat); Fat 0.8g (sat 0.1g, mono 0.5g, poly 0.1g); Protein 0.3g; Carb 4.6g; Fiber 0.7g; Chol 0mg; Iron 0.1mg; Sodium 1mg; Calc 6mg *
Substitute 1 cup each diced, refrigerated jarred mango and papaya, if desired.
Bacon-and-Egg Quesadillas
Prep: 20 min. Cook: 23 min. Instead of using the egg mixture to make quesadillas, you can also spoon it into warmed tortillas to make wraps.
Ingredients...
6 large eggs 2 tablespoons minced onion 2 tablespoons finely chopped green bell pepper 6 pickled jalapeño pepper slices, finely chopped ½ teaspoon seasoned salt ½ teaspoon seasoned pepper ½ cup Ranch dressing ½ cup salsa 1 (8-ounce) package shredded Mexican four-cheese blend 4 (10-inch) flour tortillas 4 bacon slices, cooked and crumbled ½ cup diced ham Vegetable cooking spray.
Whisk together first 6 ingredients.
Cook in a lightly greased large skillet over medium heat, without stirring, until eggs begin to set on bottom. Draw a spatula across bottom of skillet to form large curds.
Continue cooking until eggs are thickened but still moist. (Do not stir constantly.)
Remove egg mixture from skillet, and set aside. Wipe skillet clean. Stir together Ranch dressing and salsa; set salsa mixture aside.
Sprinkle ¼ cup cheese evenly onto half of each tortilla; top evenly with one-fourth of egg mixture, bacon, and ham.
Top each half with ¼ cup more cheese.
Fold tortilla in half over filling, pressing gently to seal.
Heat skillet coated with cooking spray over medium-high heat. Add quesadillas, in 2 batches, and cook 3 to 4 minutes on each side or until golden brown.
Serve with salsa mixture.
Yield: 4 servings. Per serving: Calories 796 (58% from fat); Fat 51.7g (sat 19.8g, mono 16.5g, poly 11.9g); Protein 35.7g; Carb 45.7g; Fiber 3.1g; Chol 395mg; Iron 4.5mg; Sodium 2180mg; Calc 561mg
Apple-Spinach Salad
Prep: 10 min. You can chill dressing up to one day.
Ingredients...
1 (6-ounce) package fresh baby spinach 2 small Granny Smith apples, chopped ½ cup cashews ¼ cup golden raisins ¼ cup sugar ¼ cup apple cider vinegar ¼ cup vegetable oil ¼ teaspoon garlic salt ¼ teaspoon celery salt
Directions...
Combine first 4 ingredients in a bowl. Whisk together sugar and remaining ingredients until blended.
Serve dressing with salad.
Yield: 4 servings. Per serving: Calories 346 (57% from fat); Fat 21.8g (sat 2.9g, mono 10.2g, poly 7.4g); Protein 4.2g; Carb 39.1g; Fiber 4.4g; Chol 0mg; Iron 2.9mg; Sodium 275mg; Calc 47mg
How to Have the Best Sex of Your Life(Proven ways to please an older partner)By Sandra LaMorgese AARP
Published June 06, 2018
The years after you turn 50 are a time of great change, but some of the greatest changes — ones that people don’t talk about enough — relate to sex. As our bodies and minds mature and change, our relationship to sex changes, as well. This was certainly the case for me. When I was a younger woman, my sexual experiences were primarily physical expressions of passion, lust or love.
Though aging brought many physical changes, I was surprised to realize that those changes had little effect on my ability to enjoy sex as I got older.
The reason for that is, now that I'm in my 60s, my view of sex has evolved from merely a physical expression into something more personal, intimate and even spiritual. The physicality of sex, while still important, takes a back seat to how it feels to make a connection with my partner and to be fully present in moments of intimacy.
Because of this shift, I have been able to enjoy sex more as I age, and you can, too.
All it takes is a willingness to let go of judgments and try new things to accommodate the natural physical and emotional changes of aging. It may take trial and error and the courage to venture outside of your comfort zone to reinvigorate your sex life as an older person, but this process of exploration can be powerful (and very sexy) if you approach it with patience and vulnerability.
Go with the flow
Physical changes that accompany aging can affect the amount and kind of sex you enjoy, but you can work around many of these changes by trying new methods. According to the North American Menopause Society, up to 45 percent of postmenopausal women find sex painful due, in part, to increased vaginal dryness and thinning vaginal tissue caused by falling estrogen levels.
One of the best ways to combat this extremely common natural side effect of aging is with a good-quality lubricant. Using lube is a simple and inexpensive way to make sex feel comfortable again for an older woman experiencing discomfort because of vaginal dryness. Make lube a regular part of your sex life, and when you’re with a partner, find ways to make applying it playful and sexy so that it adds to the experience.
You should also talk with your doctor or medical specialist about estrogen creams and hormone replacement therapy; both options have been shown to help vagina dryness, and they may even increase a woman’s physical and mental desire for sex.
Finally, small dietary changes may make a big difference in the bedroom for older couples. Research by the Linus Pauling Institute of Oregon State University indicates that eating plant-based foods that contain phytoestrogens may help women raise their estrogen levels. The following are examples of such foods:
Dealing with EDOlder men also experience their share of sexual challenges, the most common of which is erectile dysfunction. ED makes it difficult for a man to achieve or sustain an erection for sexual intercourse, which can be embarrassing, an ego killer and a source of serious emotional stress — all of which tend to exacerbate the symptoms of ED, resulting in a frustrating cycle.
Fortunately for men with ED (and their partners), there are numerous effective medical treatments and therapies that can help prevent this condition from becoming long term. But for those seeking a more natural remedy, the Mayo Clinic suggests lifestyle changes that may help, such as losing weight, quitting smoking, limiting alcohol intake, increasing exercise and lowering daily stress.
What's more, research from the University of Texas at Austin suggests that men can raise their testosterone levels by eating foods high in monounsaturated fat and zinc, including:
“As we age, our sexual needs and preferences may change. Where we like being touched, how we like being touched, even who we want to touch us may change. Let the changes be an opportunity to explore. Make a date with yourself or your partner to rediscover how your body responds. In a private, relaxed setting, spend a long, languid, sensual time touching without any goal except to experience sensation and pleasure. Don’t head straight to the genitals — explore your whole body. You may discover some new erogenous zones! Try different kinds of touch — slow, fast, light, firm, stroking, circling. If it feels natural, let yourself experience orgasm, but don’t put any pressure on yourself. Just enjoy learning what feels really good.”
Embrace change in sexual desire
Beyond these simple and highly effective practical strategies for making sex more physically enjoyable, I want to end where we started, talking about the most powerful thing you can do to embrace change within your relationship to sex — that is, altering your mind-set. When you free yourself from expectations based on other people or your past experiences, you empower your own happiness. This process will look different for every person and every couple, and the things you try can range from spending more time cuddling to experimenting with new positions and techniques in the bedroom. Be open and honest with yourself and your partner about what feels good and how your sexual interests and feelings may have changed over the years.
The more you practice acceptance and take pressure off yourself and your partner to perform or respond in a certain way during sex, the deeper and more satisfying your sexual experiences will become, no matter your age.
(AICR Report)
Artificial Intelligence In Cancer Care: The Future Is Now
Key Takeaways:
Artificial intelligence (AI) allows machines to learn from data to make decisions and perform humanlike tasks. It might sound like science fiction, but AI is already integrated into many aspects of health care.
Oncology stands to benefit from AI’s advances. Experts are hopeful that AI can help improve cancer research, diagnosis, personalized treatment and care.
AI in Cancer Care TodayThe most frequent uses for AI in oncology are for diagnosing and monitoring cancer. The technology helps radiologists accurately identify suspicious areas on a scan, thus reducing the chance of missing an early cancer.
Advanced AI applications also reveal cancer characteristics that are undetectable to the human eye, allowing for more targeted treatment plans. AI tools can also predict how a person will respond to various treatments.
AI Can Help with LifestyleAI tools can improve cancer survivors’ outcomes and quality of life by helping people eat better, become more physically active and manage side effects.
With funding from AICR, Dr. Katie Schmitz, a cancer researcher at the University of Pittsburgh, examined whether a tablet-based virtual assistant called Nurse AMIE (Addressing Malignancies in Individuals Everyday) could help women with metastatic breast cancer manage symptoms.
Nurse AMIE checks with users daily about their sleep, pain, fatigue, distress and activity levels. Based on the responses, Dr. Schmitz says the tool provides guidance to users on:
It also alerts the oncology team when more intensive care is needed. An initial usability and feasibility study showed users had a positive experience with Nurse AMIE. This research enabled Dr. Schmitz to secure further funding from the National Institutes of Health and National Cancer Institute for a follow-up study on the tool’s effectiveness.
Some dietitians use AI tools to help assess patients’ eating patterns and design meal plans. Patients can use AI-powered apps to accurately estimate the nutrient content of meals based on smartphone photos. Some apps even let you take photos of your food and use AI to predict the recipe ingredients with excellent accuracy. This takes the guesswork out of meal tracking and makes assessments more accurate.
AI Won’t Replace CliniciansAI technology can’t replace medical professionals. Instead, it helps improve efficiency and accuracy in their work. Virtual assistant tools like Nurse AMIE can also reach more patients for lifestyle interventions when clinics are short-staffed.
“There is a workforce shortage in nutrition, exercise oncology and cancer rehabilitation,” says Dr. Schmitz. “AI interventions could ensure that more people living with cancer have access to personalized support to improve quality of life, function and, in some cases, long-term survival.”
The Future for AI in OncologyResearchers are hopeful that soon AI tools will be able to predict where a person may develop a future cancer, shifting the focus to prevention instead of treatment. AICR is following the topic of AI in cancer prevention and treatment. The has attracted several grant applications proposing to use AI tools. Stay tuned to see how your donations will advance this innovative science.
Anne Danahy, MS, RDN
Anne Danahy, MS, RDN is a freelance health content writer and registered dietitian specializing in chronic disease prevention and management and healthy aging.
Baked Salmon With Caribbean Fruit Salsa
Prep: 5 min. Cook: 25 min. Other: 2 hrs. A whole salmon fillet makes a dramatic presentation. Ask the butcher at your grocery store to remove salmon skin.
Ingredients...
1 (3-pound) whole skinless salmon fillet 1 tablespoon Caribbean jerk seasoning* 1½ tablespoons olive oil Caribbean Fruit Salsa Garnish: lime wedges
Directions...
Place salmon fillet in a roasting pan; sprinkle evenly on 1 side with jerk seasoning. Drizzle with oil. Cover and chill 2 hours.
Bake salmon at 350° for 20 to 25 minutes or until fish flakes with a fork.
Serve with Caribbean Fruit Salsa. Garnish, if desired.
Yield: 8 servings. Per serving: Calories 346 (43% from fat); Fat 16.4g (sat 2.5g, mono 7g, poly 5.4g); Protein 39.1g; Carb 9.2g; Fiber 1.4g; Chol 108mg; Iron 1.8mg; Sodium 192mg; Calc 35mg *
Substitute Jamaican jerk seasoning, if desired. Caribbean jerk seasoning has a hint of sweetness.
Caribbean Fruit Salsa
Prep: 20 min. Other: 2 hrs.
This salsa’s also great as an appetizer served with tortilla chips.
Ingredients...
1 mango (about ½ pound), peeled and diced* 1 papaya (about ½ pound), peeled and diced* 1 medium-size red bell pepper, diced 1 medium-size green bell pepper, diced 1 cup diced fresh pineapple 1 small red onion, diced 3 tablespoons chopped fresh parsley 2 tablespoons fresh lime juice 1 tablespoon olive oil
Directions...
Stir together all ingredients. Cover and chill at least 2 hours.
Yield: 5 cups. Per ¼ cup: Calories 24 (30% from fat); Fat 0.8g (sat 0.1g, mono 0.5g, poly 0.1g); Protein 0.3g; Carb 4.6g; Fiber 0.7g; Chol 0mg; Iron 0.1mg; Sodium 1mg; Calc 6mg *
Substitute 1 cup each diced, refrigerated jarred mango and papaya, if desired.
Bacon-and-Egg Quesadillas
Prep: 20 min. Cook: 23 min. Instead of using the egg mixture to make quesadillas, you can also spoon it into warmed tortillas to make wraps.
Ingredients...
6 large eggs 2 tablespoons minced onion 2 tablespoons finely chopped green bell pepper 6 pickled jalapeño pepper slices, finely chopped ½ teaspoon seasoned salt ½ teaspoon seasoned pepper ½ cup Ranch dressing ½ cup salsa 1 (8-ounce) package shredded Mexican four-cheese blend 4 (10-inch) flour tortillas 4 bacon slices, cooked and crumbled ½ cup diced ham Vegetable cooking spray.
Whisk together first 6 ingredients.
Cook in a lightly greased large skillet over medium heat, without stirring, until eggs begin to set on bottom. Draw a spatula across bottom of skillet to form large curds.
Continue cooking until eggs are thickened but still moist. (Do not stir constantly.)
Remove egg mixture from skillet, and set aside. Wipe skillet clean. Stir together Ranch dressing and salsa; set salsa mixture aside.
Sprinkle ¼ cup cheese evenly onto half of each tortilla; top evenly with one-fourth of egg mixture, bacon, and ham.
Top each half with ¼ cup more cheese.
Fold tortilla in half over filling, pressing gently to seal.
Heat skillet coated with cooking spray over medium-high heat. Add quesadillas, in 2 batches, and cook 3 to 4 minutes on each side or until golden brown.
Serve with salsa mixture.
Yield: 4 servings. Per serving: Calories 796 (58% from fat); Fat 51.7g (sat 19.8g, mono 16.5g, poly 11.9g); Protein 35.7g; Carb 45.7g; Fiber 3.1g; Chol 395mg; Iron 4.5mg; Sodium 2180mg; Calc 561mg
Apple-Spinach Salad
Prep: 10 min. You can chill dressing up to one day.
Ingredients...
1 (6-ounce) package fresh baby spinach 2 small Granny Smith apples, chopped ½ cup cashews ¼ cup golden raisins ¼ cup sugar ¼ cup apple cider vinegar ¼ cup vegetable oil ¼ teaspoon garlic salt ¼ teaspoon celery salt
Directions...
Combine first 4 ingredients in a bowl. Whisk together sugar and remaining ingredients until blended.
Serve dressing with salad.
Yield: 4 servings. Per serving: Calories 346 (57% from fat); Fat 21.8g (sat 2.9g, mono 10.2g, poly 7.4g); Protein 4.2g; Carb 39.1g; Fiber 4.4g; Chol 0mg; Iron 2.9mg; Sodium 275mg; Calc 47mg
How to Have the Best Sex of Your Life(Proven ways to please an older partner)By Sandra LaMorgese AARP
Published June 06, 2018
The years after you turn 50 are a time of great change, but some of the greatest changes — ones that people don’t talk about enough — relate to sex. As our bodies and minds mature and change, our relationship to sex changes, as well. This was certainly the case for me. When I was a younger woman, my sexual experiences were primarily physical expressions of passion, lust or love.
Though aging brought many physical changes, I was surprised to realize that those changes had little effect on my ability to enjoy sex as I got older.
The reason for that is, now that I'm in my 60s, my view of sex has evolved from merely a physical expression into something more personal, intimate and even spiritual. The physicality of sex, while still important, takes a back seat to how it feels to make a connection with my partner and to be fully present in moments of intimacy.
Because of this shift, I have been able to enjoy sex more as I age, and you can, too.
All it takes is a willingness to let go of judgments and try new things to accommodate the natural physical and emotional changes of aging. It may take trial and error and the courage to venture outside of your comfort zone to reinvigorate your sex life as an older person, but this process of exploration can be powerful (and very sexy) if you approach it with patience and vulnerability.
Go with the flow
Physical changes that accompany aging can affect the amount and kind of sex you enjoy, but you can work around many of these changes by trying new methods. According to the North American Menopause Society, up to 45 percent of postmenopausal women find sex painful due, in part, to increased vaginal dryness and thinning vaginal tissue caused by falling estrogen levels.
One of the best ways to combat this extremely common natural side effect of aging is with a good-quality lubricant. Using lube is a simple and inexpensive way to make sex feel comfortable again for an older woman experiencing discomfort because of vaginal dryness. Make lube a regular part of your sex life, and when you’re with a partner, find ways to make applying it playful and sexy so that it adds to the experience.
You should also talk with your doctor or medical specialist about estrogen creams and hormone replacement therapy; both options have been shown to help vagina dryness, and they may even increase a woman’s physical and mental desire for sex.
Finally, small dietary changes may make a big difference in the bedroom for older couples. Research by the Linus Pauling Institute of Oregon State University indicates that eating plant-based foods that contain phytoestrogens may help women raise their estrogen levels. The following are examples of such foods:
- Seeds: flaxseeds and sesame seeds
- Fruit: apricots, oranges, strawberries and peaches
- Vegetables: yams, carrots, alfalfa sprouts, kale and celery
- Soy products: tofu, miso soup and soy yogurt
- Dark rye bread
- Legumes: lentils, peas and pinto beans
- Olives and olive oil
- Chickpeas
Dealing with EDOlder men also experience their share of sexual challenges, the most common of which is erectile dysfunction. ED makes it difficult for a man to achieve or sustain an erection for sexual intercourse, which can be embarrassing, an ego killer and a source of serious emotional stress — all of which tend to exacerbate the symptoms of ED, resulting in a frustrating cycle.
Fortunately for men with ED (and their partners), there are numerous effective medical treatments and therapies that can help prevent this condition from becoming long term. But for those seeking a more natural remedy, the Mayo Clinic suggests lifestyle changes that may help, such as losing weight, quitting smoking, limiting alcohol intake, increasing exercise and lowering daily stress.
What's more, research from the University of Texas at Austin suggests that men can raise their testosterone levels by eating foods high in monounsaturated fat and zinc, including:
- Oils: olive, canola and peanut (monounsaturated fat)
- Avocados (monounsaturated fat and magnesium)
- Olives (monounsaturated fat)
- Nuts: almonds and cashews (monounsaturated fat, zinc and magnesium)
- Oysters (zinc)
- Wheat germ (zinc)
- Shellfish: lobster and crab (zinc)
- Chickpeas (zinc)
- Oatmeal (zinc)
- Kidney beans (zinc)
- Raisins (magnesium)
- Dark green leafy vegetables (magnesium)
- Bananas (magnesium)
- Low-fat yogurt (magnesium)
“As we age, our sexual needs and preferences may change. Where we like being touched, how we like being touched, even who we want to touch us may change. Let the changes be an opportunity to explore. Make a date with yourself or your partner to rediscover how your body responds. In a private, relaxed setting, spend a long, languid, sensual time touching without any goal except to experience sensation and pleasure. Don’t head straight to the genitals — explore your whole body. You may discover some new erogenous zones! Try different kinds of touch — slow, fast, light, firm, stroking, circling. If it feels natural, let yourself experience orgasm, but don’t put any pressure on yourself. Just enjoy learning what feels really good.”
Embrace change in sexual desire
Beyond these simple and highly effective practical strategies for making sex more physically enjoyable, I want to end where we started, talking about the most powerful thing you can do to embrace change within your relationship to sex — that is, altering your mind-set. When you free yourself from expectations based on other people or your past experiences, you empower your own happiness. This process will look different for every person and every couple, and the things you try can range from spending more time cuddling to experimenting with new positions and techniques in the bedroom. Be open and honest with yourself and your partner about what feels good and how your sexual interests and feelings may have changed over the years.
The more you practice acceptance and take pressure off yourself and your partner to perform or respond in a certain way during sex, the deeper and more satisfying your sexual experiences will become, no matter your age.
(AICR Report)
Artificial Intelligence In Cancer Care: The Future Is Now
Key Takeaways:
- Cancer care can benefit from artificial intelligence (AI) tools, which can help improve cancer research, diagnosis, personalized treatment and care.
- AICR funds researchers that use AI for personalized patient support. AI can be integrated into apps that are used to track nutrition, pain levels, exercise and medications for people with cancer.
- AI technology will not replace medical professionals. Instead, it can help improve efficiency and accuracy in their work.
Artificial intelligence (AI) allows machines to learn from data to make decisions and perform humanlike tasks. It might sound like science fiction, but AI is already integrated into many aspects of health care.
Oncology stands to benefit from AI’s advances. Experts are hopeful that AI can help improve cancer research, diagnosis, personalized treatment and care.
AI in Cancer Care TodayThe most frequent uses for AI in oncology are for diagnosing and monitoring cancer. The technology helps radiologists accurately identify suspicious areas on a scan, thus reducing the chance of missing an early cancer.
Advanced AI applications also reveal cancer characteristics that are undetectable to the human eye, allowing for more targeted treatment plans. AI tools can also predict how a person will respond to various treatments.
AI Can Help with LifestyleAI tools can improve cancer survivors’ outcomes and quality of life by helping people eat better, become more physically active and manage side effects.
With funding from AICR, Dr. Katie Schmitz, a cancer researcher at the University of Pittsburgh, examined whether a tablet-based virtual assistant called Nurse AMIE (Addressing Malignancies in Individuals Everyday) could help women with metastatic breast cancer manage symptoms.
Nurse AMIE checks with users daily about their sleep, pain, fatigue, distress and activity levels. Based on the responses, Dr. Schmitz says the tool provides guidance to users on:
- Exercise
- Nutrition
- Cognitive behavioral therapy
- Sleep
- Mindfulness meditation
- Symptom management
It also alerts the oncology team when more intensive care is needed. An initial usability and feasibility study showed users had a positive experience with Nurse AMIE. This research enabled Dr. Schmitz to secure further funding from the National Institutes of Health and National Cancer Institute for a follow-up study on the tool’s effectiveness.
Some dietitians use AI tools to help assess patients’ eating patterns and design meal plans. Patients can use AI-powered apps to accurately estimate the nutrient content of meals based on smartphone photos. Some apps even let you take photos of your food and use AI to predict the recipe ingredients with excellent accuracy. This takes the guesswork out of meal tracking and makes assessments more accurate.
AI Won’t Replace CliniciansAI technology can’t replace medical professionals. Instead, it helps improve efficiency and accuracy in their work. Virtual assistant tools like Nurse AMIE can also reach more patients for lifestyle interventions when clinics are short-staffed.
“There is a workforce shortage in nutrition, exercise oncology and cancer rehabilitation,” says Dr. Schmitz. “AI interventions could ensure that more people living with cancer have access to personalized support to improve quality of life, function and, in some cases, long-term survival.”
The Future for AI in OncologyResearchers are hopeful that soon AI tools will be able to predict where a person may develop a future cancer, shifting the focus to prevention instead of treatment. AICR is following the topic of AI in cancer prevention and treatment. The has attracted several grant applications proposing to use AI tools. Stay tuned to see how your donations will advance this innovative science.
Anne Danahy, MS, RDN
Anne Danahy, MS, RDN is a freelance health content writer and registered dietitian specializing in chronic disease prevention and management and healthy aging.